Removal4
- Removal by flexible or rigid cystoscope and non-cutting grasping forceps
- Removal of INLEXZO™ by cystoscopy provides the opportunity for simultaneous assessment of disease response6
Mechanism of Delivery
Of the total gemcitabine dose, 77% was excreted by Day 7 and 99% was excreted by Day 21 in urine as gemcitabine and dFdU1
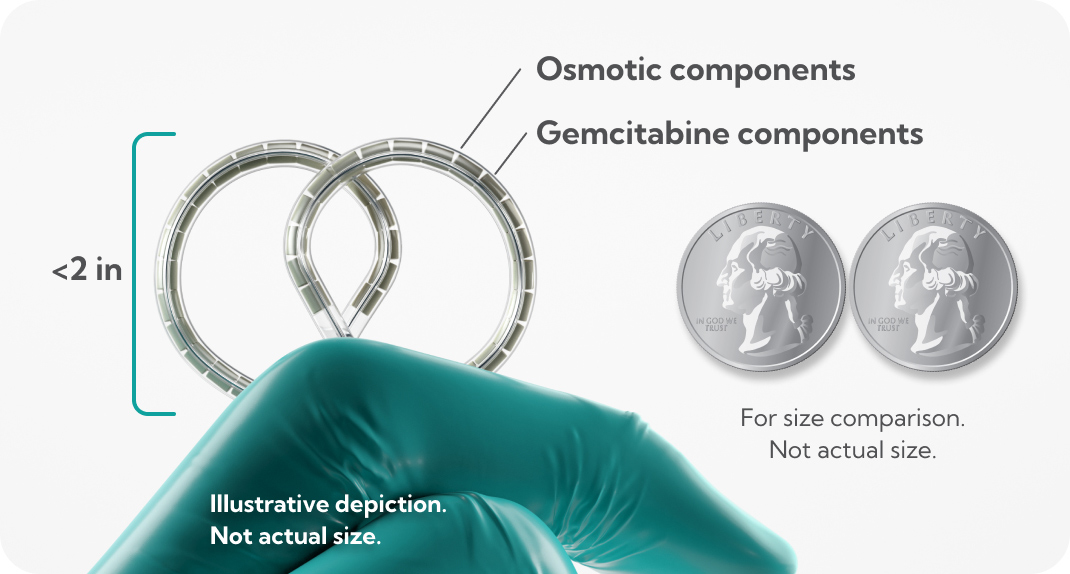
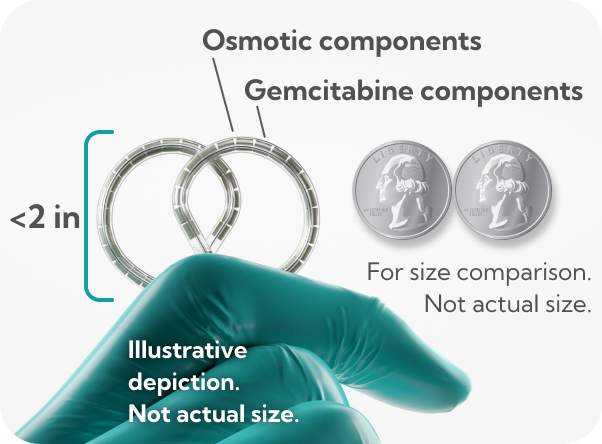
dFdU, difluorodeoxyuridine.
Illustrative depiction.
Not actual size.
INLEXZO™ Overview Video
Discover how INLEXZO™ fits into the current treatment landscape, and what makes the drug releasing system unique
Dosing and Administration
Remove INLEXZO™ after each 3-week indwelling period


†Uses catheterization and cystoscopy.4
‡Or until persistent or recurrent NMIBC, disease progression, or unacceptable toxicity.1
§Assumes same-day removal and new insertion during first 6 months.1
∥One dose=insertion and removal 3 weeks later.1


Please read full Prescribing Information for INLEXZO™ for specific MRI scanning conditions.
Read full Instructions for Use for additional handling considerations.
Please refer to Section 17 of full Prescribing Information for complete patient counseling information.
Watch the Procedural Video for INLEXZO™
For details on insertion and removal, please read full Instructions for Use.
99% (745/755) of attempted insertions were successful (N=85 patients)5
A successful insertion of INLEXZO™ was defined as obtaining transurethral access to the bladder using the urinary catheter with the ability to fully advance the stylet until INLEXZO™ was deployed in the bladder.
Disclaimer: This information is not in the Prescribing Information. It is being provided for descriptive purposes; results require cautious interpretation.
Storage and Handling
Store in the original carton at 20°-25°C (68º-77ºF)

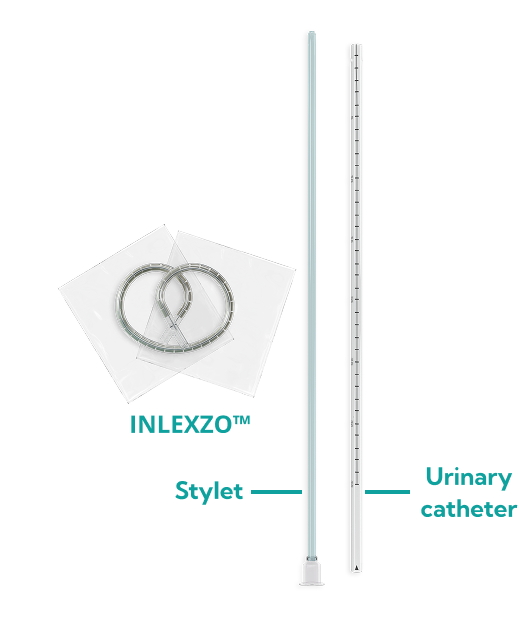
Please read the full Prescribing Information and Instructions for Use for complete information on how to prepare and administer INLEXZO™.
;